The decantation It is a physical procedure in which a solid or liquid with a higher density is separated from another that, having a lower density, occupies the upper part of a heterogeneous mixture. For example: purification of the water, elaboration of juices, the cream of the milk.
It is a widely used process in laboratories and various industrial settings, and should not be confused with sedimentation, which is the separation of solid waste into suspension by the effect of gravity over time.
For decanting to occur, the mixture must settle long enough for the denser substance to descend and can be extracted most of the time using a separating funnel.
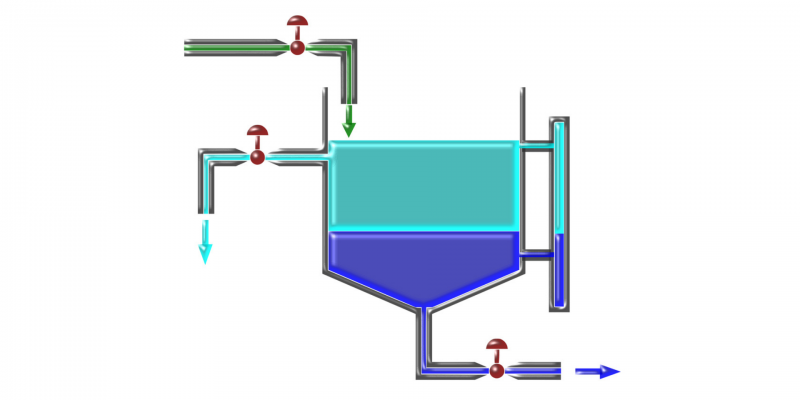
There are two ways in which it can be carried out, according to the state of aggregation of the participating substances:
- Solid-liquid settling. It is used to separate certain suspensions of solids in liquids.
- Liquid-liquid decantation. It is used to separate mixtures of two immiscible liquids. The liquid with the highest density descends to the bottom of the separatory funnel, while the other remains at the top of the mixture.
Decantation Examples

- Sewage treatment. Dirty water is usually denser than clean water since it has particles and other suspended substances, so it is possible to carry out a first filtering process through successive decantation procedures.
- Separation of oils and water. In oil extraction processes, it is often necessary to resort to decantation to separate the lipids from the water or solid residues resulting from the grinding. This is usually done through a separatory funnel.
- Separation of biodiesel and glycerin. It is a by-product of obtaining fuel from vegetable or animal fats and oils. A decantation process is often required to separate them since glycerin is much denser.
- Water purification. In the food industry, the water is usually made drinkable through decantation stages that allow the extraction of clay and suspended materials that could influence the preparation of food.
- wine decanting. To separate the liquor from the residues that may have formed in the bottle, experts recommend a decantation process, which allows the sediments to be extracted and, in the process, to oxygenate the wine. This is usual in long-matured wines.
- Preparation of the Mexican pozol. In the preparation of this fermented corn and cocoa drink, the already cooked mixture is usually decanted to separate the solid or semi-solid residues of the drink as such.
- Obtaining vinegars. During the refining processes of vinegar of vegetable origin, decantation is often used to separate it from the heavier oils obtained during the processing of the raw material.
- oil refining. Throughout the refining of oil, various types of useful hydrocarbons are obtained, both in gas and liquid form. Decantation can be used in some stages of refining where it is necessary to separate various liquids of different densities.
- Maritime oil extraction. It is also the case, when extracting oil from the seabed, that the hydrocarbon mixes with seawater, a condition that is remedied through the decantation of the hydrocarbon, which is much denser than water. The hydrocarbon is stored and the water is returned to the sea.
- in the preparation of sauces. Decanting is often used to separate substances from others throughout culinary preparation, most notably to remove unwanted fats and other liquids from a usable solution, such as sauces.
- Juice making. For example, the juice of tamarind or other fibrous fruits, in which the liquid is separated from the pulp or dense paste of the fiber, by means of simple decantation and sedimentation mechanisms.
- Ashes in a volcanic eruption. Although the ashes are very light and remain suspended in the air for a while, little by little the effect of gravity and density will make them settle, leaving the air clean again.
- Shake it before using. Many products have this recommendation on their packaging: it is due to the fact that during a certain period of rest they have been able to separate their components by the action of gravity and by differences in their densities (or by sedimentation), and only by shaking them can they recover their homogeneity.
- Recovery of mercury in aquifer contamination. Numerous accidents or practices (such as illegal mining) can release mercury into the waters of rivers and lakes, causing a lot of environmental damage. In those cases, decantation can be used to extract mercury from portions of water and try to reverse the damage.
- The cream of the milk. Due to natural settling, milk at rest separates the cream or curd (with lipid content), a yellowish and dense substance, from the rest of the milk, to the point of being able to be mechanically removed.
