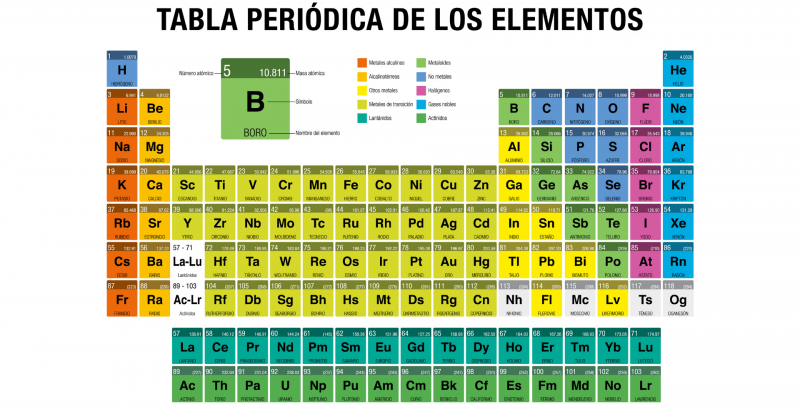
The Periodic table of elements It is a table in which all the chemical elements known to man are arranged, ordered according to their atomic number (number of protons), electron configuration and specific chemical properties.
It is a conceptual tool fundamental to the study of matter, the first version of which was published in 1869 by the Russian chemist Dmitri Mendeleev, and which has been updated over the years as new chemical elements have been discovered and the patterns underlying their properties have become better understood. properties.
The current periodic table is structured in seven rows (horizontal) called periods and in 18 columns (vertical) called groups or families. The chemical elements are arranged according to their properties from left to right throughout the periods and from top to bottom following each group.
Some properties of chemical elements, such as atomic radius and ionic radius, increase from top to bottom (following the group) and from right to left (following the period), while ionization energy, electron affinity, and electronegativity increase from bottom to top (following the group) and from left to right (following the period).
Groups of the periodic table
Numbered 1 through 18 from left to right, current group names are determined by the IUPAC nomenclature, approved in 1988 to unify the diverse forms of name that existed. The members of each group have similar electronic configurations and the same valence (number of electrons in the last orbit), so they have similar chemical properties.
According to IUPAC, there are the following groups of elements:
- Group 1 (IA). In this group are all alkali metals, with the exception of hydrogen, which although it is nominally in the group, is a gas. The elements are part of the family: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), francium (Fr). They have very low densities, are good transmitters of heat and electricity, and are never found freely in nature, but rather in compounds with other elements.
- Group 2 (IIA). In this group are the so-called alkaline earth metals, they are harder than the alkaline ones, bright and good electrical conductors, although less reactive and very good reducing agents (oxidants). The family consists of: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra).
- Group 3 (IIIB). The scandium family is included in this group, although in many of the groups in block “d” of the table (groups 3 to 12, including actinides and rare earths) there is no definitive consensus regarding the ideal arrangement. They make up this family: scandium (Sc), yttrium (Y), lanthanum or lutetium (La) and actinium (Ac), are solid, shiny and highly reactive, similar in properties to aluminum. The so-called “rare earths” also belong to this group: the lanthanides (or lanthanoids). Actinides (or actinoids) also belong to this group. Both sets of elements (the lanthanides and actinides) are called “internal transition elements” and are in a lower block of the table. Lanthanides are: Lanthanum (La), Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), Promethium (Pm), Samarium (Sm), Europium (Eu), Gadolinium (Gd), Terbium (Tb), Dysprosium (Dy), Holmium (Ho), Erbium (Er), Thulium (Tm), Ytterbium (Yb), Lutetium (Lu). Actinides are: actinium (Ac), thorium (Th), protactinium (Pa), uranium (U), neptunium (Np), plutonium (Pu), americium (Am), curium (Cm), berkelium (Bk), californium (Cf), einsteinium (Es), fermium (Fm), mendelevium (Md), nobelium (No) and lawrence (Lr). From neptunium onwards they are unstable isotopes created by man.
- Group 4 (IVB). In this group is the so-called “titanium family”, made up of the elements titanium (Ti), zirconium (Zr), hafnium (Hf) and rutherfordium (Rf), the latter synthetic and radioactive, which is why it is sometimes not taken consider. They are highly reactive metals, so in certain presentations they can instantly become red and inflamed just by coming into contact with the oxygen in the air.
- Group 5 (VB). In this group is the vanadium (V) family, headed by vanadium (V) and accompanied by niobium (Nb), tantalum (Ta) and dubnium (Db), the latter exclusively produced in laboratories. They are solid at room temperature, silver in color, and conduct heat and electricity.
- Group 6 (VIB). In this group is the chromium (Cr) family, made up of chromium (Cr), molybdenum (Mo), tungsten (W) and seaborgium (Sg). They are high-melting and boiling solids, conductors of heat and electricity, highly resistant to corrosion, and quite reactive.
- Group 7 (VIIB). In this family are manganese (Mn), technetium (Tc) and rhenium (Re), as well as the element with atomic number 107, bohrium (Bh). The latter was synthesized for the first time in 1981 and is highly unstable, so its half-life is just 0.44 seconds. Generally speaking, rhenium and technetium are also extremely rare elements: technetium does not have stable forms, while manganese is very common in nature.
- Group 8 (VIIIB). In this group is the iron (Fe) family, which includes ruthenium (Ru), osmium (Os) and hassium (Hs). Hassium was known as unniloctium and was synthesized in 1984 for the first time; it appears among the controversial elements 101 to 109, whose nomenclature has been questioned. They are quite reactive elements, good conductors of heat and electricity and, in the case of iron, magnetic.
- Group 9 (VIIIB). In this group is the family that of cobalt (Co), which contains the elements cobalt (Co), rhodium (Rh), iridium (Ir) and meitnerium (Mt). As in the previous group, the former is ferromagnetic and representative of the properties of the family, and the latter is synthetic, so it does not exist in nature (its most stable isotope lasts about 10 years).
- Group 10 (VIIIB). In earlier versions of the Periodic Table, this group was a single family, with groups 8 and 9 .. Recent versions separated them. This group is headed by nickel (Ni), which is accompanied by palladium (Pd), platinum (Pt) and darmstadtium (Ds). They are common metals in nature in elemental form, although nickel (the most reactive) can be found in alloy (in some meteorites, especially). Their catalytic properties make these metals an important supply for the aerospace and chemical industry.
- Group 11 (IB). In this group is the copper (Cu) family, and is made up of copper (Cu) and the precious metals gold (Au) and silver (Ag) and roentgenium (Rg). They are also known as “coin metals”. They are quite unreactive, difficult to corrode, soft and extremely useful to man.
- Group 12 (IIB). This group contains the zinc (Zn) family, which contains zinc, cadmium (Cd), mercury (Hg), and copernicium (Cn), formerly called ununbium. They are soft metals (in fact, mercury is the only liquid metal at room temperature), diamagnetic and divalent, with the lowest melting points of all transition metals. The funny thing is that zinc is very necessary for the chemistry of life, while cadmium and mercury are highly intoxicating. Copernicium, for its part, is a synthetic element created in 1996.
- Group 13 (IIIA). In this group are the elements known as “earthy”, since they are abundant on earth, especially aluminum. The group is headed by boron (B), which is a metalloid, and then aluminum (Al), gallium (Ga), indium (In), thallium (Ta) and nihonium (Nh), increasingly metallic as they it descends in the column. And although boron has high hardness and non-metallic properties, the others are soft and malleable metals widely used by man.
- Group 14 (VAT). In this group are the carbonid elements, headed by carbon (C), and continuing with silicon (Si), germanium (Ge), tin (Sn), lead (Pb) and flevorium (Fl). They are well-known elements, especially carbon, essential for all the chemistry of life. As one moves down the family, however, the elements acquire metallic properties, to such an extent that carbon is non-metallic, silicon and germanium semi-metal, and the latter clearly metallic.
- Group 15 (VA). In this group are the nitrogenoidal elements, headed by nitrogen (N), then phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi) and muscovium (Mc), a synthetic element . Also known as pannogens or nitrogenoids, they are highly reactive at high temperatures, and many are indispensable for organic chemistry.
- Group 16 (VIA). In this group are the so-called chalcogens or amphigens. It is in the oxygen (O) family, which contains oxygen (O), followed by sulfur (S), selenium (Se), tellurium (Te), polonium (Po) and livermorio (Lv). They are characterized by having six valence electrons, despite which their properties vary from non-metallic to metallic as their atomic number increases. At room temperature, oxygen is a very reactive gas due to its small size, while the rest of the elements are solid and less frequent in nature.
- Group 17 (VIIA). In this group is the family of halogens, a name that comes from their tendency to form salts (halides). This is due to the fact that they generally constitute diatomic molecules of considerable oxidizing power, which leads them to form mononegative ions. They are widely used in the chemical industry and in the manufacture of laboratory supplies. These elements are fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astate (At) and tenese (Ts), the latter being also a metal of group f.
- Group 18 (VIIIA). In this group are the elements known as “noble gases” or “inert gases”. They are elements of very low reactivity, which are usually found as monatomic, odorless, colorless, tasteless gases, forming very few and exceptional compounds, due to the fact that their outermost electron shell is complete. These elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganeson (Og). These last two are particular: radon is radioactive and has no stable isotopes (its most stable isotope is 222Rn, which exists only 3.8 days), while oganeson is of synthetic origin and the heaviest element created to date.
Periodic Table Blocks
Another way to understand the Periodic Table is through its four blocks:
- Block s. It comprises the first two groups, that is, alkali and alkaline earth metals, in addition to hydrogen and helium.
- Block p. It includes the last six groups, that is, from 13 to 18 in the Periodic Table and also all the metalloids.
- Block d. Includes groups 3 through 12 and all transition metals.
- Block f. It includes the rare earths (lanthanides) and actinides. It does not have its own group numbers, although it is assumed that these elements would belong to 3.
- Block g. A hypothetical block, in which the elements that can be synthesized in the future would go.
