When thinking about matter, it is usually assumed that there are three states in which it can have a form: solid, liquid and gas. These are the three most common and the so-called three states of aggregation, although there is a fourth called the “plasma state”, which can only occur under extremely high temperature, leaving ionized atoms.
The three states of aggregation conventional are those that chemistry usually analyzes, some substances, depending on the temperature to which they are subjected, can appear in all three forms at a pressure of 1 atm: the best example of this is water.
A very important question of the states of aggregation of matter is that there is the possibility that it goes from any of the three states to any other.
Aggregation state changes:
- Fusion. Matter changes from solid to liquid by increasing temperature.
- Solidification. The temperature of a liquid is lowered until it becomes a solid.
- Vaporization. Matter changes from liquid to gas by increasing temperature.
- Condensation. The temperature of a gas is lowered until it becomes a liquid.
- Sublimation. Matter is transformed from solid to gas by a sudden increase in temperature and pressure variation.
- reverse sublimation. The temperature is suddenly lowered until it becomes a solid again (in neither case does the solid turn into a liquid before turning into a gas).
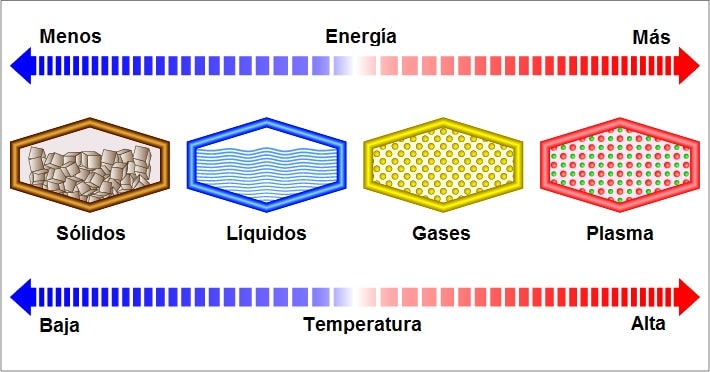
solid
The solid matter It is made up of particles that are tightly bound together. Particles can move but to a very small degree since solids do not diffuse or flow.
Characteristics of solids
- Solids are characterized by having little elasticity. Once deformed, they generally do not recover their original shape by themselves.
- The shape and volume is constant, and they cannot be compressed (their volume cannot be reduced by compression). However, certain solids tend to expand and contract; increase in volume when heated and reduce when cooled.
- The usual subdivision that is made with respect to solids is between the crystalline (which have a regular atomic structure) and amorphous (which are made up of irregularly arranged particles).
examples of solids
| Table salt | Iron | Graphite |
| Diamond | Sugar | Feldspar |
| Ice | hard plastics | And it is |
| Amber | Magnetite | Coal |
| Sulfur | Kaolin | Silicon |
| Quartz | Wood | chalcopyrite |
| pearls | Sand |
liquids
The liquids are substances that also have a narrow ordering of the particles. However, the attractive forces between particles in a liquid are weaker, so these particles move and collide with each other, vibrating and sliding past each other.
Liquid Characteristics
- Liquids can have a density similar to solids, but at the same time they adapt and flow, always having the shape of the container that contains them.
- Viscosity is a characteristic that is their own, but to a different extent depending on the case.
- Other typical properties of the liquid state is surface tension (due to the forces of attraction in all directions between the particles that compose them) and capillarity (the ease of liquids to rise through tubes of small diameter).
Examples of liquids (liquid state)
| Water | Edible oil | Benzene |
| Glycerin | Sunflower oil | Saliva |
| Acetone | Fruit juices | Chloroform |
| Milk | toluene | Petroleum |
| formaldehyde | Liquid crystal | Ethyl alcohol |
| Vinegar | phosphoric acid solution | Mercury |
| Molten metal | Cerebrospinal fluid |
gases
The gaseous state of matter is very different from the other two. The attractive forces are almost non-existent, so the particles are widely separated from each other.
Some characteristics of the gaseous state are:
- The movement made by the particles is fast and uncontrolled, and they even travel long distances. Therefore, the gas takes the size and shape of the place it occupies.
- The density of the various types of gases is much smaller than that of liquids and solids, and gases are quickly compressible.
- The gas laws (provided by Charles and Gay-Lussac) explained the most important properties of gases, referring to the relationship between pressures and temperatures of gases.
Examples of gaseous state (gases)
| Dioxygen | Dichloro | krypton |
| dihydrogen | Difluorine | Tear gas |
| Water steam | Butane | Neon |
| dinitrogen | Ozone | gas balloons |
| Argon | Methane | hydrogen sulfide gas |
| Helium | sky clouds | Ammonia |
| Carbon monoxide |
