In the field of chemistry, they are called bases (or hydroxides) to substances that, when dissolved in water, release hydroxyl ions (OH–) and are called acids substances that are capable of releasing protons (H+) in aqueous solution. For instance: sulfuric acid, nitric acid, calcium hydroxide, potassium hydroxide.
Classification of acids and bases
According to their tendency to dissociate into ions, acids and bases are classified into:
- Strong acid. It is an acid that completely ionizes in aqueous solution. For example: HCl(ac), H2SW4 (ac).
- Strong base. It is a base that completely ionizes in aqueous solution. For example: NaOH(ac), KOH(ac).
- Weak acid. It is an acid that is partially ionized in aqueous solution, so the concentration of its ions in solution is lower than that of a strong acid. For example: carbonic acid (H2CO3).
- Weak base. It is a base that is partially ionized in aqueous solution, so the concentration of its ions in solution is lower than that of a strong base. For example: ammonia (NH3).
Acids decrease pH of solutions, bases or alkalis raise it. Strong acids are often corrosive, some substances dissolve better in media that have been slightly acidified or alkalized.
Examples of acids
Some known acids are:
- Sulfuric acid (H2SW4). It is a strong acid with many uses, especially in heavy industry, highly corrosive and irritating. When diluted, it releases a lot of heat, so it must be handled (like other strong acids) with great care. It is intensely oxidizing.
- Hydrochloric acid (HCl). Although it is a strong acid, it is present in the human body, specifically in the stomach, where it plays an important role in the digestive process. Its excess generates heartburn.
- Phosphoric acid (H3PO4). This acid is a common ingredient in carbonated drinks. Regular consumption of such drinks is discouraged due to the negative impact of this acid on calcium metabolism, which affects bones and teeth above all.
- Nitric acid (HNO3). It is a recognized strong acid, used to make explosives and nitrogen fertilizers, among other uses.
- Perchloric acid (HClO4). It is a strong acid, liquid at room temperature. It is one of the most oxidizing.
- Hydrogen sulfide (H2S). It is a gaseous substance with a strong and unpleasant odor, toxic in high concentrations. It has numerous industrial applications.
- Ribonucleic acid. It is a central component of ribosomes, essential for the global process of protein synthesis from deoxyribonucleic acid to be completed.
- Acetylsalicylic acid. It is a very important organic acid, with analgesic and anti-inflammatory properties. It is the basis of aspirin.
- Lactic acid. It comes from the breakdown of glucose during anaerobic exercise of high intensity and short duration. Under normal conditions, this lactic acid is reused, but if it accumulates it causes damage to the muscle fibers, which causes cramps above all.
- Allylic acid. It is an acid present in vegetables such as garlic or onion, derived from a precursor also present in such species, allicin. It is germicidal and antioxidant.
- Retinoic acid. Applied topically, it inhibits keratinization, is used in creams against acne and skin aging. It should be used under medical supervision.
- Butyric acid. It is the end product of the fermentation of certain carbohydrates carried out by microorganisms in the rumen. It is usually part of animal fats in small amounts.
- Propionic acid. It is a food preservative, it is used in order to prevent fungal and bacterial spoilage of bakery products and others.
- Benzoic acid. It is used as a preservative added to different products (mayonnaise, canned goods), often in the form of salt (sodium benzoate).
- Acetic acid (CH3COOH). It is a food preservative widely used in the home, also as a base for vinaigrettes and pickles. It is the majority component of vinegar.
- Hydroiodic acid (HI(ac)). It is a strong acid that can be used to increase the levels of iodine in salts.
- Succinic acid (C4H6OR4). It is a crystalline solid that can be obtained from amber. It can be generated in the fermentation process of wine and beer.
- Hydrobromic acid (HBr(ac)). It is a very corrosive strong acid. Its reaction with bases is very violent, it is also very irritating. It is used in the chemical and pharmaceutical industry.
- Citric acid (C6H8OR7) It is an organic acid abundant in fruits. It is a natural antioxidant.
- Oxalic acid (H2C2OR4). It is an organic acid that is naturally found in plants. It is used in beekeeping to control diseases in bees. It is also used to make cleaning products, in the textile industry, among other uses.
Examples of bases
Metal bases are known generically as hydroxides. Some bases are:
- Sodium hydroxide (NaOH, caustic soda). It is a strong base that is used in the paper industry and in the manufacture of detergents. In everyday life it is used to unclog bathroom and kitchen pipes.
- Magnesium Hydroxide (Mg (OH)2, milk of magnesia). It is a strong base that is sometimes used as an antacid or laxative.
- Calcium hydroxide (Ca (OH)2, lime). Also known as hydrated lime, it is used in the metallurgical and oil industry. It is also used to make pesticides, in the sugar and dairy industries among others.
- Potassium hydroxide (KOH). It is a strong and corrosive base that is widely used in different industries. It is widely used for making soap.
- Barium hydroxide (Ba (OH)2). Due to its toxicity, it is used to make poisons. It is also used in the ceramic industry, in the paper industry and in the process of refining sugar.
- Iron II or III hydroxide (Fe (OH)2 or Fe (OH)3). It is usually generated as part of the metallurgical industry. It is used in the manufacture of paints among other uses.
- Ammonia (NH3). It is a gas with a characteristic odor. It is used to make fertilizers and many drugs. It is very dangerous if inhaled in high doses.
- Soap. It is a sodium or potassium salt. It is used for personal and general hygiene.
- Detergent. It is also a widely used product for hygiene.
- Quinine. It is a natural base produced by some plants. It has antipyretic and analgesic properties. In ancient times it was used to treat malaria.
- Aniline. It is a toxic compound when ingested or inhaled. It is used in the rubber industry, in the manufacture of herbicides and explosives, among others.
- Guanine. It is one of the nitrogenous bases that are part of nucleic acids (DNA and RNA).
- Pyrimidine. The nitrogenous bases that make up nucleic acids are derived from pyrimidine.
- Cytosine. It is one of the nitrogenous bases that are part of nucleic acids.
- Adenine. It is one of the nitrogenous bases that are part of nucleic acids.
- Zinc hydroxide (Zn (OH)2). It is an amphoteric substance (it can act both as an acid and as a base). It is a toxic substance if it comes into contact with the eyes or the skin. It is used in the manufacturing process of surgical dressings.
- Copper hydroxide (Cu (OH)2). It is used as a fungicide and to color ceramic objects. It is also used as a catalyst for some chemical reactions.
- Zirconium Hydroxide IV (Zr (OH)4). It is used in the ceramic and glass industry.
- Beryllium hydroxide (Be (OH)2). It has amphoteric properties. It is used in industry to obtain metallic beryllium. It is a substance of limited abundance.
- Aluminum hydroxide (Al (OH)3, antacid). It is used in medicine as an antacid and adjuvant to vaccines.
Theories about acids and bases
The concept of bases and acids has been changing over time. It was Arrhenius who drew up the first definition, which defines an acid as a substance that yields H ions in aqueous solution+, and a base as a substance that in aqueous solution gives up OH ions–. His theory had some limitations, since certain substances (such as ammonia) behave like bases without having the hydroxyl ion in their molecule.
Furthermore, Arrhenius only considered substances in aqueous media, but acid-base reactions also occur in other non-aqueous dissolution media. A representation of an acid and a base according to the Arrhenius theory is:
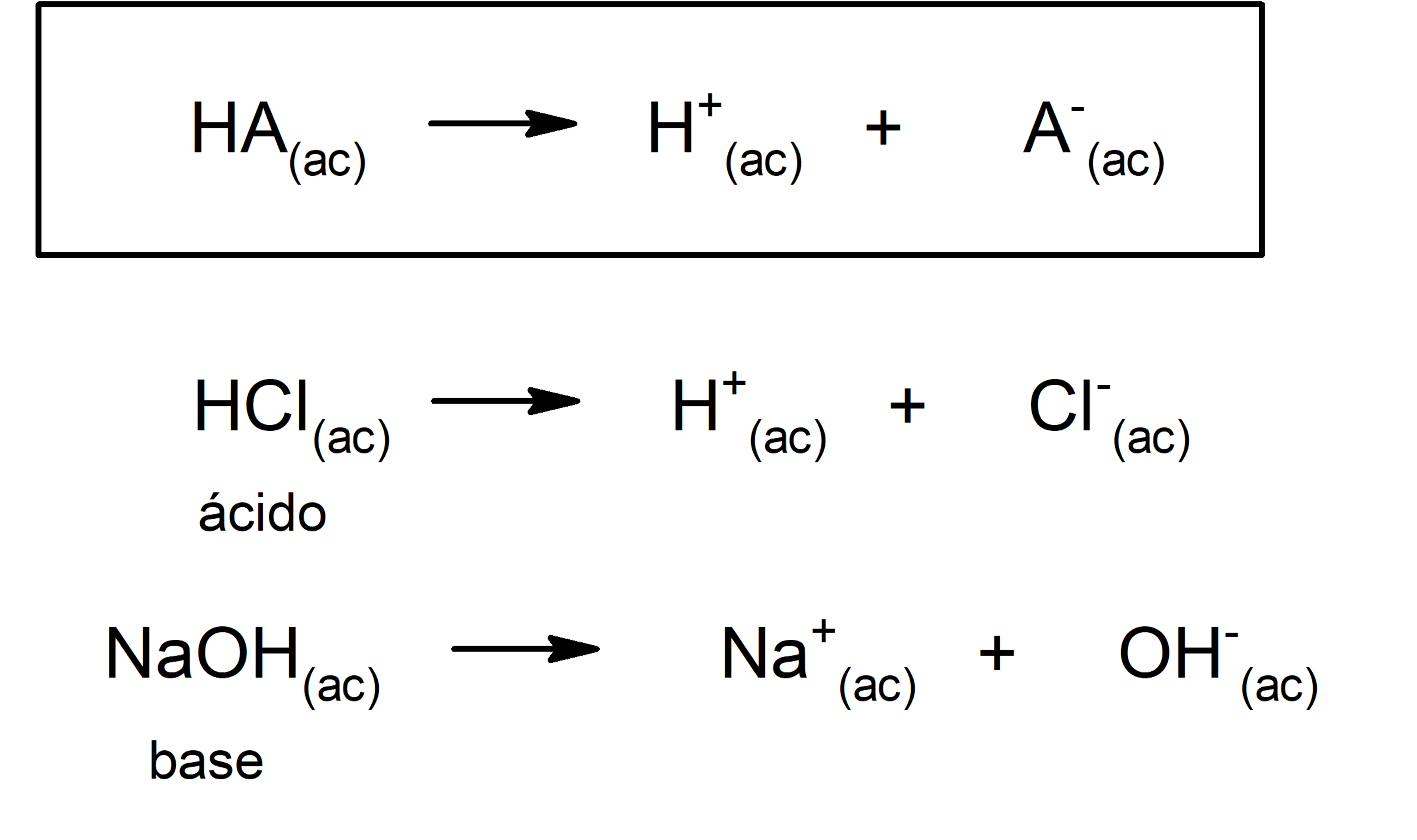
Almost forty years later, around 1923, Brönsted and Lowry formulated another theory by stating that acids and bases act as conjugated pairs. According to this theory, the acid is that substance capable of giving up protons (in this case it does not refer to the protons of the atomic nucleus, but to the cations H+, being H+ an abbreviation for cation H3OR+) and the base is that substance capable of accepting those protons.
This theory states that in an acid-base reaction, a conjugate base is the chemical species that forms after an acid donates a proton, and a conjugated acid is the chemical species that forms after the base accepts a proton. This theory is not entirely complete, since there are several substances that have acidic properties without having ionizable hydrogen atoms in their structure.
But on the other hand, in this theory it is not mandatory that the substances exist in aqueous solution. A representation of an acid (and its conjugated base) and a base (and its conjugated acid) according to the Brönsted – Lowry theory is the protonation of ammonia, which does not have to occur in aqueous medium:
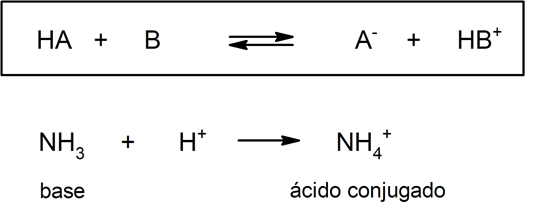
Therefore, as an additional part of his theory on covalent bonding, Lewis developed a theory in which he defines an acid as any substance that can accept a pair of electrons, while a base is any substance capable of giving up said pair. electronic.
According Lewis, the concepts of acid and base do not involve gain or loss of OH ions– and H+Instead, it proposes that H + itself is the acid (it can accept electrons) and OH- is the base (it can donate electrons). A representation of an acid-base reaction according to Lewis theory is:

Where OH- (which belongs to NaOH) donates the unshared electron pair to H + (which belongs to HCl), as a result a coordinate or dative link (covalent bond in which the shared pair of electrons is contributed by only one of the atoms involved in the bond) to form the water molecule.
