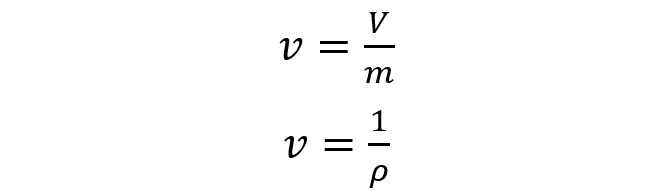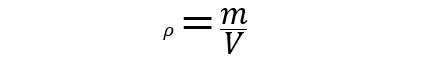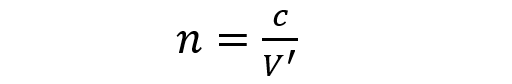The matter it is the substance of which something is made. For example, the material of a nail can be iron. The matter of living things is organic matter.
When we talk about matter we refer to something that has mass and volume, that is, it occupies a space.
Matter has two types of properties:
- Extensive properties. They depend on the mass. For instance: weight, inertia, volume.
- Intensive properties (or intrinsic). They do not depend on mass, that is, they remain unchanged. For instance: pressure, density, flavor.
There are some extensive properties that can be used as intensive. For example, volume is a extensive property. However, it can become a intensive property if it is considered as a value per unit mass or quantity of substance, such as molar volume (the volume of one mole of the substance).
Examples of intensive properties

- Temperature. It is the amount of heat in a substance. It is measured in degrees. For instance: This water sample has a temperature of 32 ºC. The example does not specify the amount of water because the intensive properties do not change with the amount. If the sample is two liters at 32 º Celsius Also, the temperature will be the same as if the sample is 200 cm3.
- Boiling temperature. Also called the boiling point, it is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid. It is the maximum temperature that a substance can reach in a liquid state before it becomes completely a gas. If the substance exceeds that temperature, it will be in a gaseous state. For instance: the boiling temperature of water is 100 ° C.
- Melting point or melting point. It is the temperature at which a substance goes from a solid to a liquid state. In general, the melting point is equal to the freezing point (for example, for water, the melting point and the freezing point is 0 ° C). However, there are some exceptions such as agar-agar, which has a melting point of 85 ° C and freezes between 31 and 40 ° C. For instance: the melting point of silver is 961 ° C.
- Pressure. It is a physical quantity that measures the projection of force in a perpendicular direction per unit area. It is usually measured in pascals. When atmospheric pressure is measured (the pressure exerted by the atmosphere on the earth) it is measured in hectopascals (hPa), where 1 hPa is equal to 100 pascals. For instance: the pressure today in this locality is 1013 hPa.
- Specific volume (v). Although volume is an extensive property, specific volume is an intensive property because it is volume (V) occupied by a unit of mass (m) of a material. It is the inverse magnitude of the density. It is measured in units of volume times a unit of mass (for example, cubic meters per kilogram). For instance: The specific volume of water at 20 ° C is 0.001002 m3/ kg.

- Density (). It is the magnitude of the amount of mass (m) in a certain volume (V). That is, the density of a body is the ratio between the mass of a body and the volume it occupies. For instance: the density of sunflower oil is 0.891 g / cm3.

- Colour. It refers to how a substance looks to the human eye. For instance: The color of the wood can be orange, brown or coppery.
- Flavor. In chemistry, one rarely works with the taste of substances since many of them are toxic. However, it is important to remember that it is one of the intensive properties of substances. For instance: the taste of lemon is sour.
- Compressibility. It is the capacity of matter to decrease in volume when subjected to a certain pressure or compression.
- Concentration. In a solution, the concentration is the ratio between the amount of solute (the substance in the smallest proportion, usually a solid) and the amount of solvent (the substance that dissolves). The greater the amount of solute compared to that of solvent, the more concentrated the solution is said. The smaller the amount of solute compared to that of solvent, the more dilute the solution is said to be.
- Refractive index (n). It is the quotient between the speed of light in vacuum (c) and the speed of light in the substance of which we calculate the index (V ‘). That is, the faster the light passes through that substance, the lower the refractive index. The refractive index of vacuum is 1, the refractive index of air is 1.0002926, the refractive index of diamond is 2.42.

- Surface tension. It is a property of liquids. It is the ability of some liquids to prevent their surface from increasing. Surface tension is the force acting tangentially per unit length at the edge of a surface of a liquid in equilibrium. The surface tension causes water droplets to form and the water does not spread over an entire surface. Water has a surface tension of 72.75 x 10-3 N / m (Newton / meter), while other liquids have lower surface tensions, such as acetone (23.70 x 10-3 N / m) or ethyl alcohol (22.75 x 10-3 N / m).
- Elasticity. It is the ability of some materials to return to their original shape after having suffered deformations as a result of the application of an external force.
Examples of extensive properties
- Weight. It is a measure of strength. It is the gravitational force that acts on an object. The weight of a body on Earth will have a value, while the weight of the same body on the Moon will be much less, while its mass will remain the same. It is a vector magnitude.
- Mass. Represents the resistance of a body to change in motion. It is also a property related to the acceleration of a body. It is important not to confuse mass with weight. To measure it, the kilogram and its equivalent units are used. It is a scalar quantity.
- Volume. It is the extension of an object in three dimensions. It is a quantity derived from the length. The most widely used units of volume are the liter and cubic centimeters (cm3). A liter is 1,000 cm3.
- Potential energy. Within a physical system, the potential energy of an object is the energy stored according to its position under the influence of a force field, which can be gravitational, electrostatic, among others. For example, a brick hanging from a rope two meters high has the potential energy of the gravitational field. Since potential energy depends on weight, mass, and volume, it is an extensive property.
- Inertia. Inertia is the property of an object to remain in a state of rest or of uniform rectilinear motion. Any state of rest (immobility) or movement is always relative, since it depends on the point of view of the observer.
- Length. In the same way that volume changes with the amount of matter, so does length. Length is measured in only one dimension, as opposed to volume which is measured in three (length, width, depth).
- Heat capacity. It is the amount of heat that allows the temperature of a body to vary by one degree. It depends on the amount of substance since, for example, it takes more heat to heat a liter of water than half a liter of water.