The iron it is one of the most abundant metals in the earth’s crust, second after aluminum. However, on a planetary level, it is the most abundant metal, since it is found in high concentrations in the Earth’s core.
The color of the iron is silver gray. In nature it is rarely free, but forms minerals and many oxides.
Iron uses
Iron represents 95% of world metal production. It has been used by humans for at least six thousand years. Usually it is not used in its pure state, except for its magnetic use, but rather as a matrix element for alloys with other metals and with non-metals.
- One of the main uses of iron is to form steel, which is iron with a maximum percentage of 2.1% carbon.
- Iron with carbon and 12% chromium forms stainless steel.
- The molten iron, or gray iron, is an iron alloy with more than 2% carbon (therefore not steel) and more than 1% silicon. Manganese, phosphorus and sulfur are also found in its composition.
- The wrought iron or sweet iron It is the type of iron that can be forged and hammered to take different shapes when subjected to high temperatures, which is often called “red-hot”. If it cools quickly, wrought iron hardens. This type of iron has much less carbon than steel (between 0.05% and 0.25%).

Iron and health
Iron is an element necessary in the body, since it is part of proteins and is necessary for the oxygenation of tissues and the metabolism of cells. However, an excess of iron is harmful to health.
For that reason, cast iron water pipes are no longer being manufactured. Excess iron deposition in tissues is called siderosis. Furthermore, work in the iron and steel foundry has been shown to be a major cause of cancer.
Iron extraction

Since iron is not found in its pure state in nature, it is obtained mainly from oxides and other minerals.
Most of iron reserves in mines they are found in Australia, second in Brazil, in Russia and in China. To extract iron from mines, explosives are first used to dislodge the rock. The material obtained is transported in trucks to the treatment plant.
However, prior to arrival at the treatment plant, trucks pass through metal detectors to check that iron is in your load. If not, the load is discarded. Once the load reaches the plant, the rocks are crushed.
The shredded material is separated into those that contain metal and those that do not, through one of two methods:
- Floatation. Detergents are used that adhere to the iron and make it float.
- Magnets. They attract only iron and not rocks.
The selected fragments still have less than 70% iron.
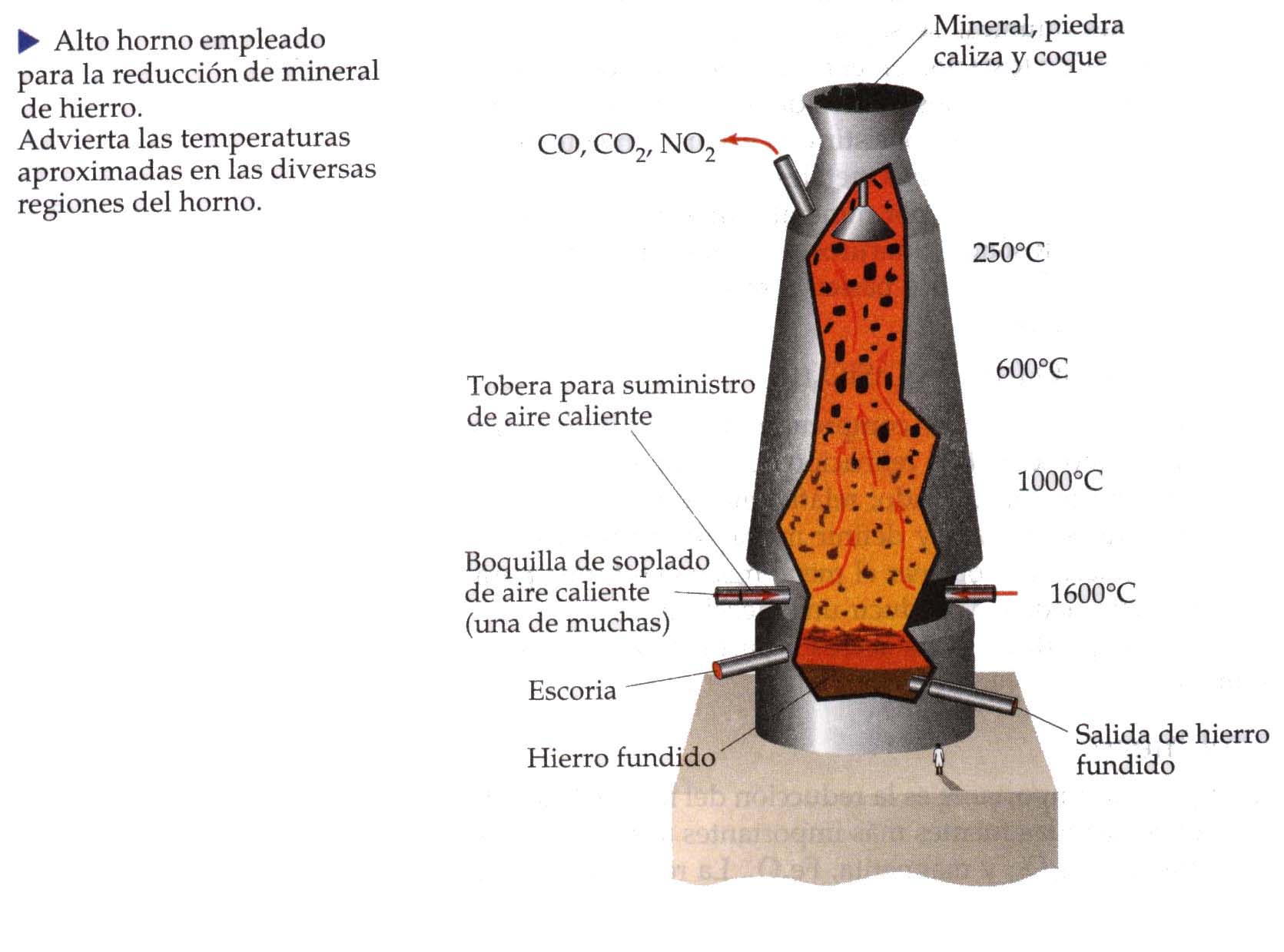
For purify them, are located in a blast furnace, where they reach high temperatures together with coke and calcium carbonate. As the temperature increases, gases are formed that allow carbon to react with oxygen, forming carbon dioxide, and later carbon monoxide.
Iron oxides bind to carbon monoxide, forming carbon dioxide and releasing the iron molecule. Once the temperature drops, the sulfur is removed through the air inlet. Of all the substances that entered the oven, two differentiated fractions are formed:
- Slag. Mix of oxides and sulfides.
- Pig iron. Substance with impurities but with a high percentage of iron.
The pig iron is then subjected to new high temperatures in converter furnaces. In these furnaces the necessary gases are injected to produce steel, cast iron, wrought iron, or any other alloy.
