There are several theories to define acids and bases, including Arrhenius, Brönsted-Lowry, and Lewis.
- According to Arrhenius theory. An acid is a substance that, when in aqueous solution, gives up hydronium ions (H3OR+) or (H+), while a base is a substance that in aqueous solution gives up hydroxyl ions (OH–). For example: hydrochloric acid (HCl) and sodium hydroxide (NaOH) they are an Arrhenius acid and base respectively.
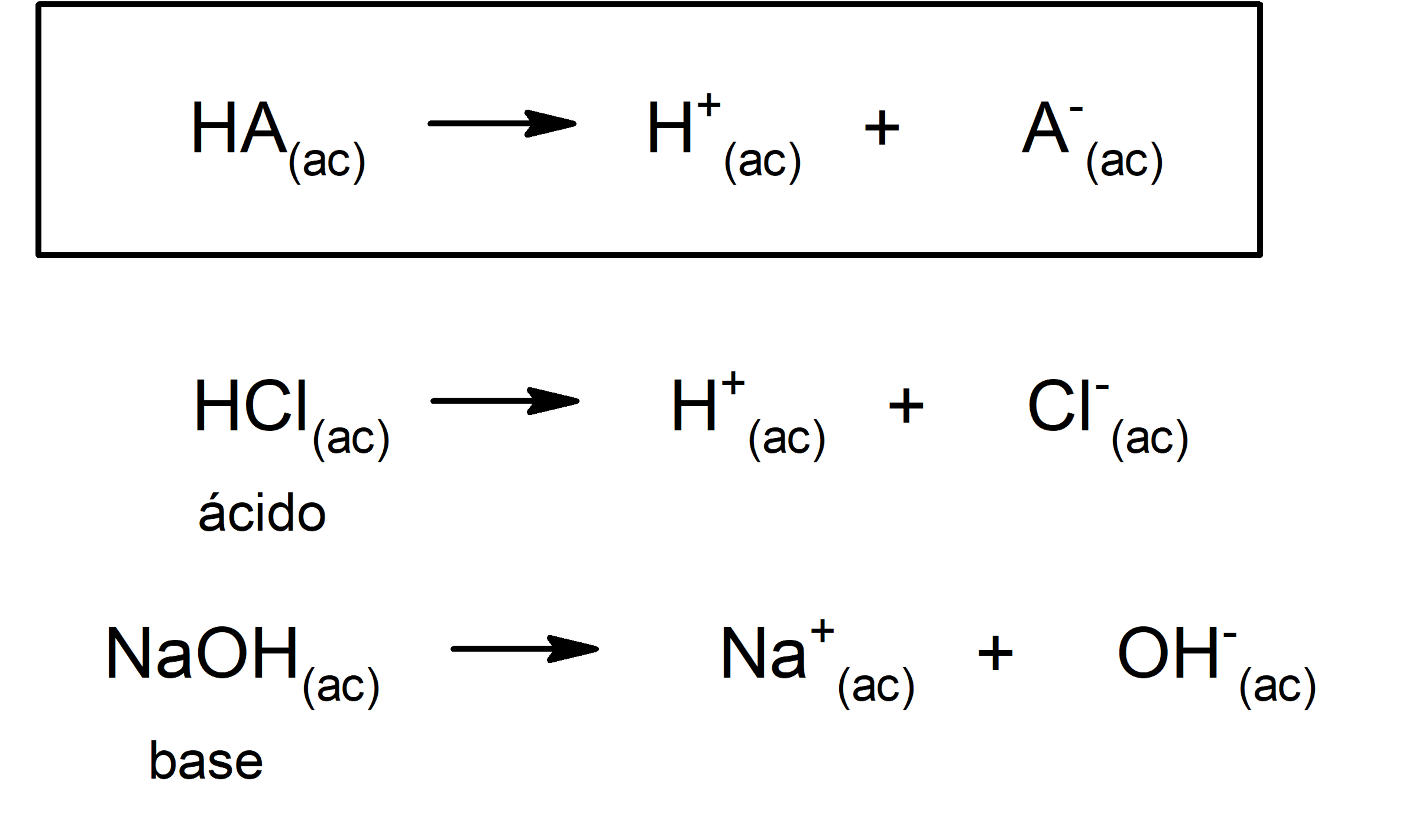
- According to the Brönsted-Lowry theory. An acid is a chemical species that gives up a hydronium ion to another chemical species (Brönsted-Lowry base), while a base is a chemical species that accepts a hydronium ion from another chemical species (Brönsted-Lowry acid). For example: acetic acid (CH3COOH) and ammonia (NH3) they are an acid and a Brönsted-Lowry base respectively.
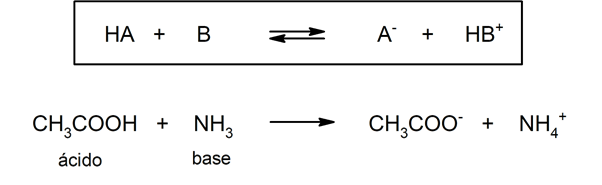
- According to Lewis’s theory. An acid is a chemical that accepts an electron pair from another chemical (Lewis base), while a Lewis base is a substance that gives up an electron pair to another substance (Lewis acid). For example: boron trifluoride (BF3) and the fluoride ion (F–) they are a Lewis acid and a base respectively.
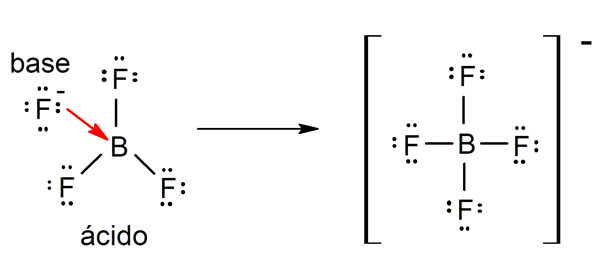
Type acids oxacids They are usually formed by the reaction between a non-metallic oxide with water, while acids of the hydracid type are formed by the combination of a non-metal with hydrogen in aqueous solution. For example: sulfuric acid (HtwoSW4) it is an oxacid and hydrochloric acid (HCl(ac)) it is a hydracid.
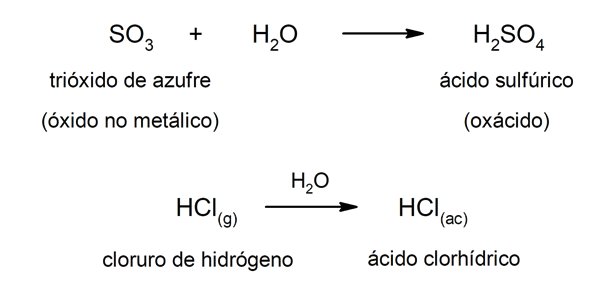
On the other hand, bases can be formed as a result of the reaction between a metal oxide and water. For example: magnesium hydroxide (Mg (OH)two).
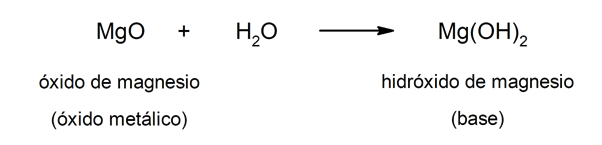
General characteristics of acids and bases
In general, acids are sour and corrosive. Bases are also corrosive, bitter tasting, caustic when in contact with the skin, and have a soapy touch. On the other hand, acid solutions have a pH lower than 7, while base solutions have a pH higher than 7.
Acid and base strength
The tendency of an acid to dissociate and lower the pH is often referred to as “acid strength.” An acid is strong when it can fully dissociate in aqueous solution and is weak when its dissociation occurs partially. Examples of strong acids are perchloric (HClO4), sulfuric (HtwoSW4), hydroiodic (HI), hydrobromic (HBr), hydrochloric (HCl) and nitric (HNO3). On the other hand, acetic acid (CH3COOH), citrus (C6H8OR7) and benzoic (C6H5COOH) are weak.
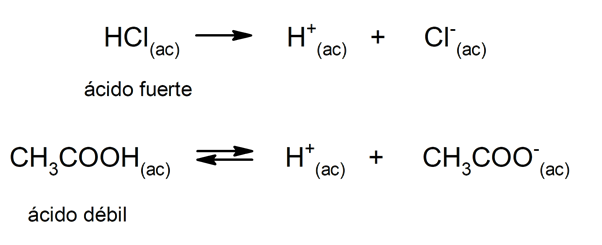
Similarly, they can be considered as strong bases those that dissociate completely in aqueous solution, and weak when their dissociation occurs partially. Examples of strong bases are potassium hydroxide (KOH), sodium (NaOH), lithium (LiOH) and magnesium (Mg (OH)two). On the other hand, ammonia (NH3) is a weak base.
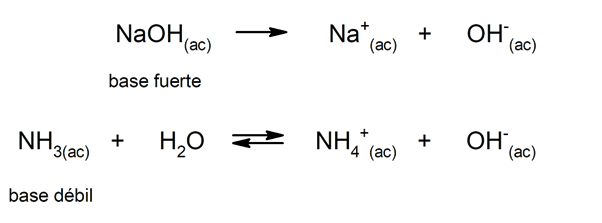
How are salts formed?
The you go out They are ionic compounds of various complexity, abundant in nature and are generally formed by the combination of acids with bases in a neutralization reaction, which generates a release of water. They can also be formed as a result of the reaction between a metal and an acid, a metal and a nonmetal, or the reaction between different salts.
The salts can be classified into:
- Neutral or binary salts. They are made up of only one metal and one nonmetal. For example: sodium chloride (NaCl) Y potassium bromide (KBr).
- Acid salts. They are formed when the hydrogens of an oxacid are partially substituted. For example: sodium hydrogen carbonate (NaHCO3), a hydrogen is substituted for carbonic acid (HtwoCO3).
- Oxacid or ternary salts. They are formed when all the hydrogens in an oxacid are replaced. For example: magnesium sulfate (MgSO4), all the hydrogens of sulfuric acid (HtwoSW4).
- Double salts. They are formed when two hydrogens of an oxacid are replaced by two different cations. For example: double sodium potassium sulfate (KNaSO4), two hydrogens of sulfuric acid (HtwoSW4).
- You go out basic. They are formed by the hydroxide ion (OH–) and other anions. For example: copper (II) trihydroxide chloride (CutwoCl (OH)3).
Distribution and importance
Acids are very important both in industry like in the nature. For example, hydrochloric acid is part of our digestive system and is necessary for us to break down the nutritional compounds present in food. Deoxyribonucleic acid, better known as DNA, makes up the chromosomes, which is where the genetic information necessary for living things to multiply and develop is encoded. Boric acid is a prominent component in the glass industry.
The calcium carbonate It is a very abundant salt in various types of limestone rocks. By action of high temperatures (900 ° C) of calcium carbonate, calcium oxide or quicklime is obtained. Adding water to quicklime produces calcium hydroxide, called slaked lime, which is a base. These materials are used in construction.
