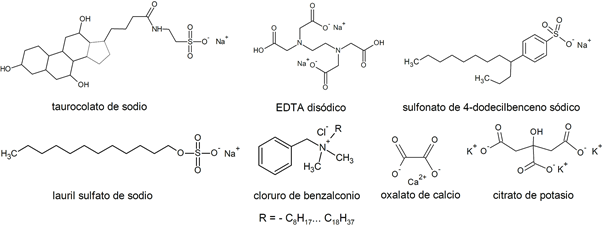The chemical combination of certain cations with certain anions originates what in chemistry is called you go out. In nature there are numerous salts dissolved in the seas or precipitated, forming minerals of medium or high complexity. In general, these are mineral salts, that is, made up of inorganic elements. For instance: calcium oxalate, sodium chloride, copper sulfate.
The salts often have a crystal structure, although in liquid media they are dissolved and ionized. Salts are essential for living beings, they help maintain cellular homeostasis and osmotic balance due to their ability to absorb water molecules. a liter of sea water it contains on average about 33 to 39 grams of salt. Many salts have important domestic uses, others are essential for industry or construction.
However, a excess salts it can lead to serious health problems, such as high blood pressure. Some living things need mineral salts to form protective external structures, such as shells and shells. Furthermore, the bones of all vertebrates are made up of salts.
Where is the salt obtained from?
In nature, many salts are concentrated in the salt flats, that is, in surface lakes whose sediments are composed of salts. When evaporating by the action of the sun in the water, the salts accumulate.
They are famous in America, for example, the Salar de Uyuni in Bolivia or Atacama in Chile. There are also endorheic seas or lakes that are very rich in salts: the best known example is that of the Dead Sea, located 416.5 meters below sea level, especially rich in calcium, magnesium, potassium and bromine salts. The salt content of the Dead Sea is around 28%.
Organic salts are the result of the reaction between an alkali, such as sodium or potassium hydroxide, and a fatty acid. This process is known as saponification. Organic salts often have detergent properties, so they are often the basis for various cleaning products.
There are several chemical reactions that lead to the formation of salts, such as:
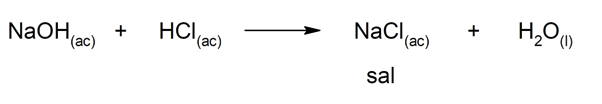
- Reaction between a base (or alkali) and an acid:
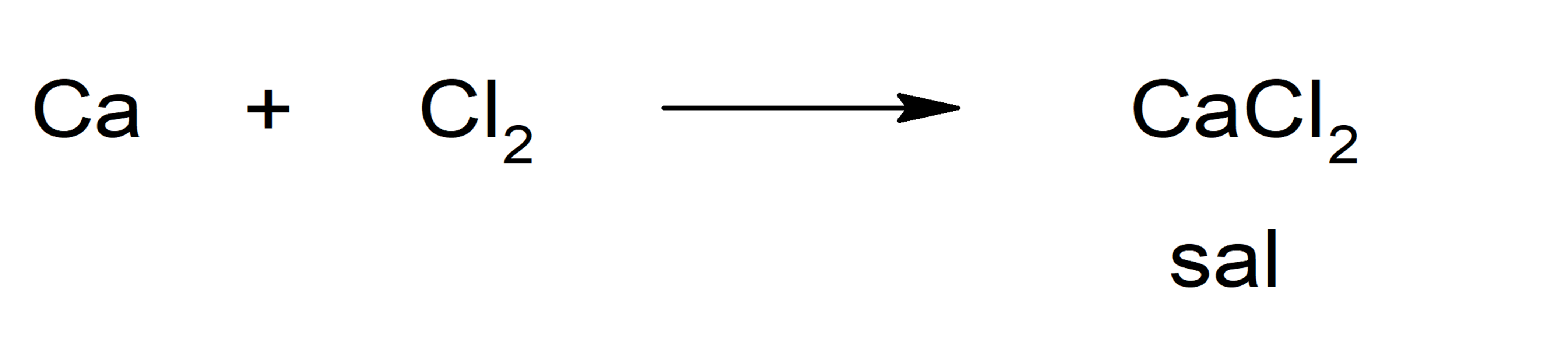
- Reaction between a metal and a nonmetal:
- Reaction between a metal and an acid:
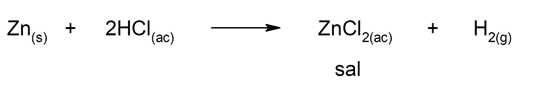
- Saponification reaction:
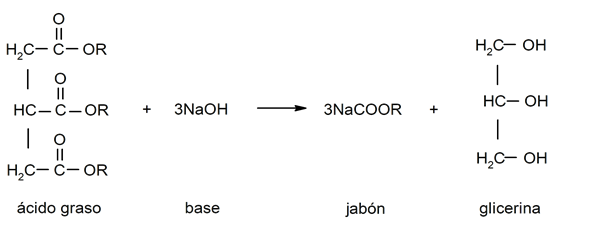
Examples of salts
| Sodium taurocholate | Benzalkonium chloride |
| Calcium carbonate (CaCO3) | Calcium oxalate |
| Potassium bromide (KBr) | NaHCO sodium bicarbonate3 |
| Magnesium sulfate (MgSO4) | Disodium EDTA |
| Sodium Chloride (NaCl) | Magnesium carbonate (MgCO3) |
| Sodium hypochlorite (NaClO) | Copper sulfate (CuSO4) |
| Tricalcium phosphate (Ca3(PO4)2) | Potassium citrate |
| Ammonium chloride (NH4Cl) | Ammonium sulfate ((NH4)2SW4) |
| Sodium lauryl sulfate | Sodium tellurite (Na2TeO3) |
| Sodium 4-dodecylbenzene sulfonate | Potassium permanganate (KMnO4) |