The diffusion it is a physical process that is based on the flow of particles of a chemical species from a region of higher concentration of this species to a region of lower concentration. This process will occur until the concentration in both regions is the same.
Generally, diffusion occurs in liquids and gases. For example, him aroma of a meal When entering a room it is a consequence of the diffusion of a mixture of two gases, that is, the gaseous particles of the food (the aroma) penetrate into the room, which initially only contained air. In this way, they diffuse until the concentration of these particles is the same outside and inside the room.
Some characteristics of a broadcast:
- It occurs from a higher solute concentration to a lower solute concentration zone.
- It is a gradual process, since the particles of the substance that is diffused can undergo various modifications while the process occurs. In addition, it is a type of passive transport, that is, it is not necessary to apply external energy for it to occur.
- The speed of diffusion will depend on the mass and size of the particles of each substance. There are heavy gases whose diffusion is slower and others (lighter gases) whose diffusion is faster.
Examples of diffusion

- The perfume of flowers in a closed place.
- A scent that spreads throughout a room.
- A scented person who walks into a room and everyone can smell his perfume.
- The smoke given off by cars on the road.
- Smoke from chimneys in homes or factories.
- The smell of rotting food in the refrigerator.
- The smell of a scented candle, incense or match.
- The smoke of a cigarette in an airtight room.
- Aromatic essences.
- The smell of a rotten egg in a container.
Effusion
The effusion It is the process by which a gas escapes to the outside of a container through a small opening or crack. The effusion rate is directly proportional to the average velocity of the gas particles.
This means that if a heavy gas molecule presents an effusion, it will do so more slowly than a lighter gas molecule, in which case the effusion will be faster. For instance: deflated balloon.
Graham’s Law
If the pressure and temperature conditions are equal, the rate of diffusion and effusion of gases is inversely proportional to the square roots of their molar masses.
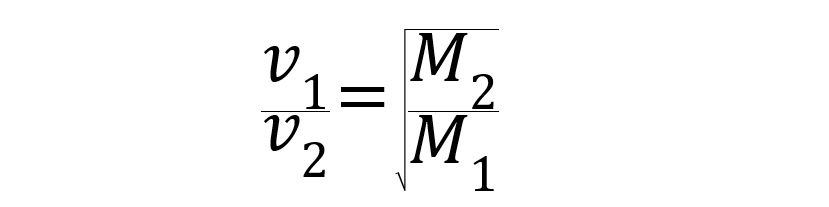
Being v1 and v2 the velocities of gases 1 and 2 expressed in meters per second (m / s), and M1 and M2 the molar masses of gases 1 and 2 expressed in kilograms per mole (kg / mole).
Examples of effusion

- Push the button on a deodorant.
- Turn a burner knob to turn it on or off.
- A helium canister with a leak.
- A hot air balloon that presents a leak.
- Powered backpacks.
- The gas tubes of the astronauts.
- A balloon deflating.
- A flatulence.
- The separation of uranium-238 into uranium-235-
- A gas cylinder with a small leak through which this gas travels to another compartment or to the outside.
