The ductile materials They are those capable of plastic deformation and sustainability, without breaking or violating its structure. For instance: wood, zinc, lead, aluminum.
Ductile materials are the opposite of brittle materials (they fracture very easily when deformed). But they should not be confused with malleable materials either (they deform easily without breaking when compressed).
This does not mean that ductile materials cannot break; in fact, they do, but after having suffered notorious deformations. Nor does it mean that ductile materials are soft; the force necessary for its deformation must be considerable, and in the face of weak forces so will its change in shape.
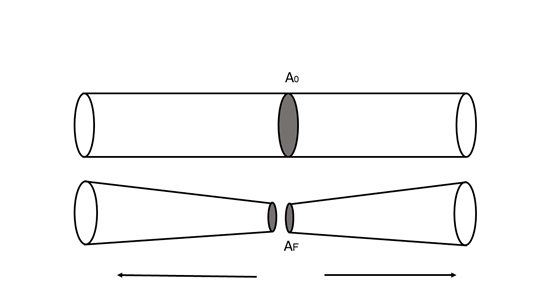
Furthermore, the deformation of ductile materials can increase in the presence of heat, without reaching the limits of the melt. One of the ways to calculate or measure the ductility of a material is by applying a traction force at two of its ends, and then calculating in percentage how much the cross-sectional area decreased in the area where it fractured:
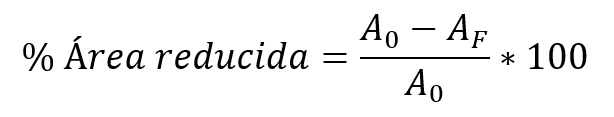
The latter are the most common ductile materials, since their atoms are configured in such a way that they can slide over each other, allowing the obtaining of wires and threads of different thicknesses.
Ductile materials are valued in the metallurgical industry and tool making, as they can take specific shapes before breaking. However, the insistent and repeated deformation will lead to fatigue in some areas of the metal and to its breakage, further evidenced by the increase in temperature of the area on which the deforming force affects.
A characteristic of ductile materials is that the relationship between the longitudinal elongation caused by the application of a tensile force and the decrease in cross-sectional area is very large. Therefore, smaller fibers or yarns can be obtained from a larger piece.
Examples of ductile materials

- Iron. Also called iron and represented by the chemical symbol Fe, it is the fourth most abundant element in the earth’s crust and the most abundant in planetary mass because the planet’s core is made up of iron and nickel in liquid state, which when moving generate a powerful magnetic field. It is a gray, malleable metal with magnetic properties and extreme hardness and density. Therefore, in its pure state, this prevents it from being useful, so it is alloyed with carbon to obtain the family of steels, which according to the proportion of this element present may be more or less ductile and more or less resistant.
- Wood. It is a fairly ductile organic material, depending on its nature and the percentage of moisture present in it, as well as the location of the knots it contains. However, being fibrous, it can be easily opened by forces perpendicular to its grain.
- Steel. It is called with this name to a mixture of iron and carbon (up to 2.14%) whose composition produces a hard and relatively ductile material, especially combined with boron to form wires of superficial hardness and very high ductility, or in steel corrugated used in the construction sector. This makes it ideal to resist weights without fracturing the concrete and allowing minimal deformations according to the weight dimension.
- Zinc. Zinc (Zn) is an essential element for life that in its pure state enjoys high ductility and malleability. It can be rolled into sheets, stretched and deformed, but the presence of minimal contaminants from other elements is enough to make it brittle and brittle. It is essential in alloys such as that produced by brass.
- Lead. This metallic element of the periodic table, with the symbol Pb, was not recognized at the time as metallic due to its enormous molecular elasticity. It is a heavy, grayish, flexible and easily meltable metal. It is used today as a cable cover, since its unique ductility makes it extremely appropriate, as it can be stretched to suit the needs to be covered.
- Brass. It is an alloy of copper (70%) and zinc (30%), characterized by its very high ductility. It is an ideal material for the manufacture of containers and containers, as well as tools that do not require extreme hardness. Combined with tin, it is resistant to rust and saltpeter, in addition to acquiring malleability.
- Clay. Extremely ductile, this plastic substance composed of calcium, petroleum jelly and aliphatic compounds, was invented in 1880. Usually made of colors and associated with the world of children’s learning, it is characterized by its ability to be deformed without breaking, allowing its simple work with the hands. , instruments or any type of surface.
- Copper. It is a bright reddish transition metal that, together with gold and silver, are the best metallic conductors of electricity. That is why it is the preferred metal when building electrical cables and both electrical and electronic components, since it is also economical, malleable and ductile.
- Platinum. This heavy, malleable and ductile grayish-white transition metal is valued in jewelry and laboratories as being corrosion resistant and precious in nature. It is also common to find platinum (Pt) in catalytic additives for automobiles, electrical contacts and other types of applications that take advantage of its resistance.
- Aluminum. Aluminum (Al) is a non-ferromagnetic metallic element and the third most common in the earth’s crust. It is highly used in the materials industry, although it can be extracted as a metal only from bauxite, due to its properties such as low density, high conduction of heat and electricity, high resistance to corrosion, economic cost and alloability. That is why it was the most widely used metal in the 20th century, along with steel. Although its natural ductility does not appear to be extreme, in certain foundry alloys its ductility, stress and corrosion are reinforced, usually through the incorporation of Silicon (5 to 12%) and magnesium.
