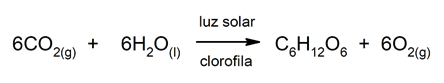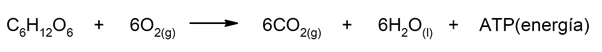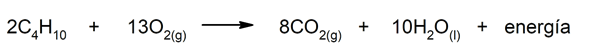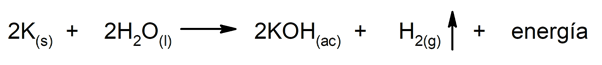The chemical energy It is the one that can be released in the different chemical reactions to which matter is susceptible, that is, that contained in the different chemical compounds. For instance: photosynthesis, explosions, batteries and cells.
Chemical energy it is used daily in various areas of our life in which various chemical reactions take place. It is often said that this form of energy is contained in bodies, and for that very reason it will become apparent to us only when these bodies are subjected to some significant alteration in their matter.
In fact, all forms of fuel contain chemical energy that can be translated into a quantity of heat, which can be converted into a certain work. And in that sense, any source of chemical energy can release its energy during the transformation of matter in which it was contained.
Examples of chemical energy
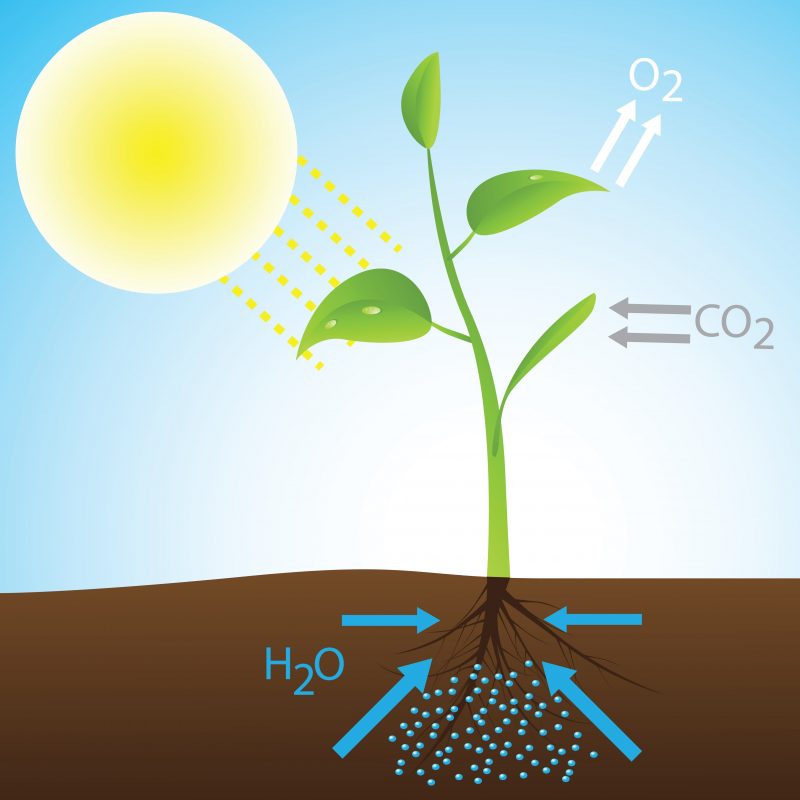
- Photosynthesis. Plants obtain their energy from the chemical reaction that takes place inside them, between sunlight, CO2 and water, and form glucose (C6H12OR6) and oxygen (O2). This energy product of a chemical reaction is contained in the molecules of the participating substances and is used by plants for their benefit and vital maintenance.

- The breathing. Animals require oxygen and glucose to release water, CO2 and obtain energy, essential to maintain the cycle of life. This is one of the processes that keeps us alive and that we share with the entire animal kingdom.

- The combustion. When we start a motor vehicle, the gasoline or the mixture of hydrocarbons that it uses as fuel is subjected to a cycle of controlled combustions and detonations that generates the energy that, in turn, allows movement. This fuel contains this energy in the hydrocarbon molecules that during the reactions are transformed into other compounds and release energy. Butane is one of the hydrocarbons that make up gasoline:

- The descomposition. Fungi and bacteria that feed on decaying organic matter can obtain the energy necessary for their fermentation processes from sugars and starches, and obtain alcohols or other products as a result of the process that breaks down organic matter molecules. This is similar to what happens in our stomach, where acids break the molecular bonds of the molecules that make up food, generating calories.
- Space travel. The fuels used by ships that travel to the moon or send satellites into space are not ordinary, like those consumed by an internal combustion engine. Rather, they are the result of highly complex chemical reactions whose release of energy is so great that it can counteract the law of gravity on an object the magnitudes of a rocket long enough to leave the atmosphere.
- Corrosion. Many of the chemical substances that we handle in our daily lives, such as drain cleaners and others that contain extreme acids or bases, are corrosive materials, capable of wearing down the surface with which they come into contact, in a process that releases heat and consumes all the organic material. Many burns from contact with corrosives are due to the heat produced by the breakdown of lipids in the skin, rather than the effect of the substance itself.
- Exothermic reactions. Many substances (such as caustic soda) are so drying that when they come into contact with water, they react exothermically, that is, they release heat. These reactions, which are not exclusive to strong bases, release energy into the environment and can be dangerous to living things.

- Explosions. There are very chemically unstable substances that, when they come into contact with oxygen in the air, react releasing large and sudden amounts of heat energy and generate an explosion.
- Batteries and batteries. The batteries we use so much (remote controls, cars, cell phones) contain various acids and metals in controlled reaction, the immediate result of which is a usable amount of electricity. When the batteries expire, this electricity is lost and the batteries must be replaced.

Other types of energy
| Potential energy | Mechanical energy |
| Hydroelectric power | Internal energy |
| Electric power | Thermal energy |
| Chemical energy | Solar energy |
| Wind power | Nuclear energy |
| Kinetic energy | Sound energy |
| Caloric energy | Hydraulic energy |
| Geothermal energy | |